Multiple Choice
A mixture of two enantiomers with a composition of 65.0% R has an observed rotation of -25.3° in a 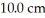 polarimeter tube. If the mixture has a concentration of 2.038 g/mL at 25°C, what is the predicted
polarimeter tube. If the mixture has a concentration of 2.038 g/mL at 25°C, what is the predicted 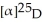
Of an optically pure sample of the S enantiomer?
A) -25.3°
B) -53.5°
C) +12.4°
D) +41.3°
E) +53.5°
Correct Answer:

Verified
Correct Answer:
Verified
Q48: Is the molecule shown below chiral or
Q49: (-)-Lactic acid has a specific rotation of
Q50: Assign the proper configurational label, R or
Q51: Which of the following terms best describes
Q52: Label each asymmetric carbon in the molecule
Q54: How many asymmetric carbon atoms are present
Q55: Label each asymmetric carbon in the compound
Q56: The boiling point of S-2-butanol is 99°C.
Q57: How many enantiomers are there of the
Q58: How many diastereomers are there of the