Multiple Choice
Which of the statements below correctly describes the chair conformations of 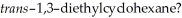
A) The two chair conformations are equal in energy.
B) The higher energy chair conformation contains two axial ethyl groups.
C) The higher energy chair conformation contains two equatorial ethyl groups.
D) The lower energy chair conformation contains two axial ethyl groups.
E) The lower energy chair conformation contains two equatorial ethyl groups.
Correct Answer:

Verified
Correct Answer:
Verified
Q115: When one compares the densities of n-hexane
Q116: A branched alkane has _ boiling point
Q117: Identify the correct IUPAC name for the
Q118: Draw the Newman projection of the highest
Q119: A series of compounds, like the n-alkanes,
Q121: Draw the most stable conformation of trans-1,4-dipropylcyclohexane.
Q122: Place the following alkanes in order of
Q123: Provide an acceptable name for [(CH<sub>3</sub>)<sub>3</sub>C]<sub>2</sub>CHCH<sub>3</sub>.
Q124: Which of the following statements regarding cyclobutane
Q125: Name the alkane shown.<br>[(CH<sub>3</sub>)<sub>2</sub>CH]<sub>2</sub>CHCH<sub>3</sub>