Multiple Choice
Given the following pKa values, what is the major ionization state of histidine at pH 11?
(α-COOH = 2.0, α-NH3+ = 9.0 and R-group imine = 6.5)
A) 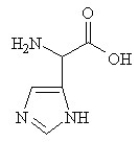
B) 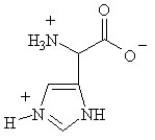
C) 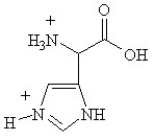
D) 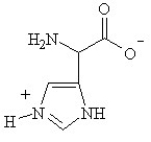
E) 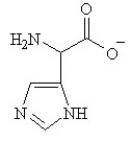
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q27: Provide a reasonable synthesis of racemic alanine
Q28: A student proposed to make aspartic acid
Q29: How would you make tryptophan using the
Q30: Which of the following compounds would reduce
Q31: What is the major force responsible for
Q33: Which of the following arrangements is usually
Q34: Provide the structure of the predominant form
Q35: How many standard amino acids are there?<br>A)
Q36: Amino acids have _.<br>A) high melting points
Q37: Provide the structure of the predominant form