Essay
Although F is more electronegative than Cl, the C-Cl bond has a larger dipole moment than the 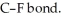
Explain.
Correct Answer:

Verified
The bond dipole moment is determined by ...View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Correct Answer:
Verified
The bond dipole moment is determined by ...
View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Related Questions
Q90: What type of solvent is best for
Q91: Which of the following is a vicinal
Q92: Provide the structure of 1-bromo-3-methylhexane.
Q93: Which of the following statements correctly describe(s)
Q94: Which of the following alkyl chlorides would
Q96: Name one factor that is favorable for
Q97: Complete the following S<sub>N</sub>2 reaction by providing
Q98: When 1-bromo-2, 2-dimethylcyclopentane is heated in ethanol,
Q99: How many distinct alkene products are possible,
Q100: What is Zaitsev's rule?