Multiple Choice
What is the hybridization of the nitrogen atom in the molecule below? 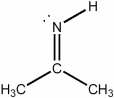
A) s
B) sp
C) sp2
D) sp3
E) none of the above
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q78: Choose the correct hybridization for the atom
Q79: The compounds below are base pairs used
Q80: The molecule shown below contains _ pi
Q81: In the structure below, the sigma bond
Q82: What is the approximate value of the
Q84: What two hybrid atomic orbitals overlap to
Q85: Shown below is one of the sex
Q86: Which of the molecules below has the
Q87: Which atomic orbital combination would result in
Q88: Which compound is more soluble in water?