Multiple Choice
Which of the following best describes the relationship between the two structures shown? 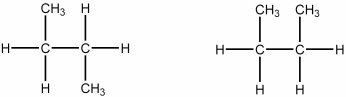
A) They represent the same compound.
B) They represent different compounds that are constitutional isomers.
C) They represent different compounds that are geometric isomers.
D) They represent different compounds that are alkenes.
E) They represent different compounds that are alkanes.
Correct Answer:

Verified
Correct Answer:
Verified
Q69: What hybrid atomic orbitals are overlapping to
Q70: Draw the hydrogen bonding that takes place
Q71: Which of the following functional groups does
Q72: If a compound, C<sub>5</sub>H<sub>7</sub>NO, contains 1 ring,
Q73: What kind of molecular orbital (σ, σ<sup>*</sup>,
Q75: Are the two compounds shown below best
Q76: Based on the structure below, what is
Q77: Which of the following compounds is not
Q78: Choose the correct hybridization for the atom
Q79: The compounds below are base pairs used