Essay
When toluene is treated with bromine (shown below), the bromine doesn't react with the double bond to form a viscinal dihalide, but instead a substitution of one of the benzylic hydrogens takes place. Why? 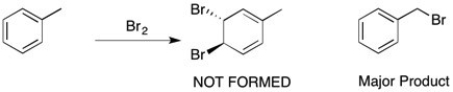
Correct Answer:

Verified
Addition of Br2 to the double bonds of be...View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Correct Answer:
Verified
View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Q9: Which of the following compounds may correctly
Q10: Is the molecule below aromatic, antiaromatic, or
Q11: Provide the structure of sodium cyclopentadienide.
Q12: Classify the compound below as aromatic antiaromatic,
Q13: Classify cyclopropenyl cation as aromatic, antiaromatic, or
Q15: Is the molecule below aromatic, antiaromatic, or
Q16: Provide an acceptable name for the compound
Q17: When cyclohexene is treated with KMnO<sub>4</sub>, H<sub>2</sub>O,
Q18: Circle and name the aromatic heterocycles in
Q19: Describe the occupied π molecular orbitals in