Essay
Even assuming the molecule below is planar, it would still be classified as non-aromatic. Why? 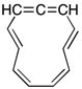
Correct Answer:

Verified
The center carbon of the allen...View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Correct Answer:
Verified
The center carbon of the allen...
View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Related Questions
Q81: Classify cyclopentadiene as aromatic, antiaromatic, or nonaromatic.
Q82: Classify cycloheptatriene as aromatic, antiaromatic, or nonaromatic.
Q83: Nitrogen's lone pair electrons occupy what type
Q84: Classify the compound below as aromatic, antiaromatic,
Q85: Classify the compound below as aromatic, antiaromatic,
Q87: How many peaks are in the proton
Q88: Classify the compound below as aromatic, antiaromatic,
Q89: Why would the reaction below proceed at
Q90: How could you distinguish the two compounds
Q91: Which of the following is also an