Essay
Styrene (shown below) has 8 π electrons but is aromatic. Why? 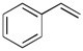
Correct Answer:

Verified
Only 6 π electrons a...View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Correct Answer:
Verified
Only 6 π electrons a...
View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Related Questions
Q44: Provide the structure of anthracene.
Q45: How many pairs of degenerate π molecular
Q46: The IR spectrum of m-xylene contains stretches
Q47: Provide an acceptable name for the compound
Q48: Classify cycloheptatrienyl cation as aromatic, antiaromatic, or
Q50: Which orbital is the lone pair of
Q51: Use the polygon rule to draw the
Q52: Provide the major resonance structures of the
Q53: Classify the following compound as aromatic, antiaromatic,
Q54: Provide an acceptable name for the compound