Essay
Use resonance structures to explain why the compound shown below is considered to be antiaromatic. 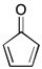
Correct Answer:

Verified
 The cycli...
The cycli...View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Correct Answer:
Verified
View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Q113: Provide a diagram which depicts the relative
Q114: Classify the compound below as aromatic, antiaromatic,
Q115: Show how the p orbitals overlap to
Q116: Classify the compound below as aromatic, antiaromatic,
Q117: Cyclic hydrocarbons which can be represented as
Q119: Provide the structure of m-xylene.
Q120: Correctly name the following compound. <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB6199/.jpg"
Q121: Which of the following compounds has the
Q122: Provide the structure of m-iodobenzoic acid.
Q123: The MO energy diagram for cyclobutadiene is