Short Answer
Classify the compound below as aromatic, antiaromatic, or nonaromatic. Assume planarity of the π network. 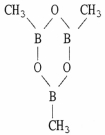
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q92: What is suggested by the fact that
Q93: Classify the compound below as aromatic, antiaromatic,
Q94: Is the molecule below aromatic, antiaromatic, or
Q95: Is the molecule below aromatic, antiaromatic, or
Q96: Is the molecule below aromatic, antiaromatic, or
Q98: Classify each nitrogen atom in the compound
Q99: Which of the following is not a
Q100: Which sequence correctly ranks the following substrates
Q101: In the molecular orbital representation of benzene,
Q102: Classify naphthalene as aromatic, antiaromatic, or nonaromatic.