Short Answer
Classify the compound below as aromatic, antiaromatic, or nonaromatic. Assume planarity of the π network. 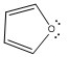
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q70: Provide the structure of m-nitrophenol.
Q71: Classify pyridine as aromatic, antiaromatic, or nonaromatic.
Q72: Rank the following in order of increasing
Q73: Which of the following is another name
Q74: What is the bond order of the
Q76: How many distinct nodal planes which are
Q77: Provide the structure of anisole.
Q78: Which of the following is the same
Q79: Which is more stable, cyclobutadiene or 1,3-butadiene?
Q80: Provide an acceptable name for the compound