Multiple Choice
According to the 1,3-butadiene structure below, which positions would be best to place methoxy groups to yield the most reactive dimethoxy-1,3-butadiene isomer in the Diels-Alder reaction? 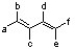
A) c and d
B) a and c
C) b and c
D) a and d
E) a and f
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q39: Provide the structure of the major organic
Q40: How many electrons are present in the
Q41: Would you expect the following compound to
Q42: In the allyl radical, which π molecular
Q43: Draw the structure of the major product
Q45: Draw structures for the two major products
Q46: Draw the resonance structures of the intermediate
Q47: Which of the following compounds is the
Q48: Indicate whether the following cyclization, conducted under
Q49: Show how the carbon p orbitals overlap