Essay
One or more of the atoms in the structure shown should have nonzero formal charges. Add the correct formal charge/s. (All non-bonding electrons have been included.) 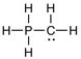
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q46: One or more of the atoms in
Q47: Strong bases usually contain positively charged atoms
Q48: Within a given row of the periodic
Q49: How many distinct p orbitals exist in
Q50: What is the molecular formula for the
Q52: For most compounds in which a nitrogen
Q53: Which of the following pairs of bases
Q54: Which sequence correctly ranks the following protons
Q55: The Lewis structure of pentane is shown
Q56: Draw a correct Lewis structure for tert-butyl