Short Answer
Predict the number of signals expected (disregarding splitting) in the 1H NMR spectrum of the compound shown below. 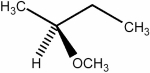
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q17: How might the two trimethylcyclohexane isomers shown
Q18: Given that compound X has a molecular
Q19: Predict the number of signals expected in
Q20: Deduce the identity of the following compound
Q21: Which carbon signal is furthest down field
Q23: Deduce the identity of the following compound
Q24: Predict the number of signals expected in
Q25: The chair form of cyclohexane has protons
Q26: If a signal is observed in the
Q27: Using a 60 MHz spectrometer, the protons