Solved
Which One of the Following Represents the Lowest Energy -Bonding Molecular Orbital of 1,3-Butadiene?
A)I
B)II
C)III
D)IV
Multiple Choice
Which one of the following represents the lowest energy -bonding molecular orbital of 1,3-butadiene? 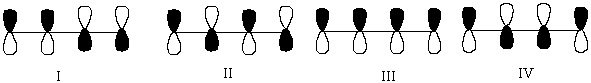
A) I
B) II
C) III
D) IV
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q14: Provide the structure of the 1,4 addition
Q32: Predict the major product for the following
Q34: Which of the following compound(s)have the
Q38: Predict the major product for the following
Q39: Which one of the following represents the
Q42: What is the correct classification of the
Q62: How many electrons does the HOMO of
Q79: Which is the most energetically favorable
Q83: A thermodynamically-controlled reaction will yield :<br>A) the
Q128: How many electrons does the HOMO of