Essay
The free radical polymerization of styrene with benzoyl peroxide yields a polymer that has repeat units arranged primarily in a 'head-to-tail' arrangement.This means that the phenyl group primarily ends up placed at alternating carbon atoms along the chain.Use correct arrow formalism to show why this arrangement is preferred over a 'head-to-head' or 'tail-to-tail' arrangement. 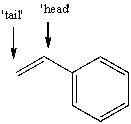
Correct Answer:

Verified
The 'head-to-tail' addition gi...View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Correct Answer:
Verified
View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Q18: Which of the following processes is responsible
Q33: Use correct arrow formalism to show the
Q36: Upon treatment with NBS and irradiation with
Q38: Which term best describes the process shown
Q40: A bromine radical can add to the
Q41: Which intermediate leads to the major product
Q42: What term most accurately describes the process
Q68: How many constitutional isomers are possible if
Q70: One possible product of thermal cracking of
Q86: Use correct arrow formalism to show the