Multiple Choice
Could water protonate the following compound? 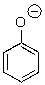
A) Yes
B) No
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q24: Into which of the following categories does
Q25: Why is acetic acid more acidic than
Q26: Can this base exist in water? <img
Q27: For the following reaction,draw the mechanism. <img
Q28: Which side will the following acid-base reaction
Q30: Could the amide anion (<sup>- </sup>NH<sub>2</sub>)deprotonate the
Q31: Which is the best descriptor for the
Q32: Identify the acid and the base and
Q33: Can this base exist in water? <img
Q34: Is the indicated compound acting an acid