Multiple Choice
What problem is encountered in the synthesis of ethyl acetoacetate (shown below) if sodium methoxide is used rather than sodium ethoxide? 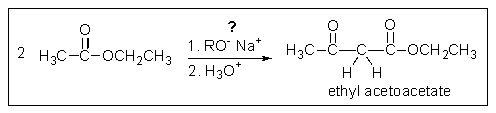
A) Sodium methoxide is too weak a base to cause this reaction to occur.
B) The condensation proceeds further to give tri-carbonyl products.
C) The reaction would produce some of both the methyl ester and the ethyl ester.
D) Sodium methoxide is too insoluble in methanol to promote the reaction.
E) Sodium methoxide is not basic enough to drive the reaction to completion.
Correct Answer:

Verified
Correct Answer:
Verified
Q19: Predict the major organic product of the
Q20: Which of the following reaction sequences will
Q21: What missing reactant would be required to
Q22: Which of the following processes involves loss
Q23: What factors contribute to the stability of
Q24: Which of the following compounds will not
Q25: Predict the major product of the following
Q26: Predict the major product of the following
Q28: What would result from the reaction shown
Q29: Why is the following reaction typically done?