Multiple Choice
What statement is true of the following energy diagram? 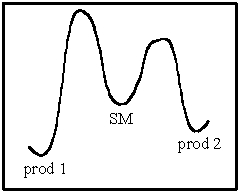
A) For irreversible reactions,product 1 will dominate.
B) For reversible reactions,product 2 will dominate at equilibrium.
C) For reversible reactions,equal amounts of 1 and 2 will be formed.
D) For irreversible reactions,equal amounts of 1 and 2 will be formed.
E) None of the above are true.
Correct Answer:

Verified
Correct Answer:
Verified
Q24: What is the correct IUPAC name for
Q25: Guanacastepene is a natural product that was
Q26: Which of the following reactions has a
Q27: Which of the following is not considered
Q28: Rotation around the carbon-carbon bond of the
Q30: The common name for 1-methylpropyl is:<br>A) Isopropyl<br>B)
Q31: What is the correct IUPAC name for
Q32: Which of the following molecules contain both
Q33: How many quaternary carbons are in the
Q34: What is the smallest alkane that is