Multiple Choice
The lone-pair of electrons on nitrogen in the following molecule reside in what type of orbital? 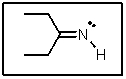
A) sp3
B) sp2
C) sp
D) 2p
E) 2s
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q4: A hydrocarbon with a double bond and
Q5: Which structure is different from the others?<br>A)<br><img
Q6: The nitrogen of trimethylamine [(CH<sub>3</sub>)<sub>3</sub>N] contains how
Q7: In the following molecule,how many carbon atoms
Q8: Which of the following molecules are most
Q10: Of those indicated,which would be the shortest
Q11: Camptothecin is an important anticancer compound; how
Q12: In the following molecule,how many carbon atoms
Q13: In the following molecule,how many carbon atoms
Q14: Which of the following pairs are not