Multiple Choice
The molecular formula C2H4O can be converted into three-line bond (Kekulé) structures that are consistent with valence rules. Which one of the following Kekulé structures is not consistent with valence rules?
A) 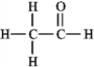
B) 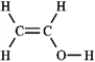
C) 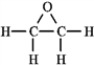
D) 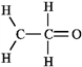
Correct Answer:

Verified
Correct Answer:
Verified
Q4: Consider the formation of an sp<sup>2</sup> hybrid
Q5: Specify the hybridization of each carbon atom
Q6: Draw all possible structures of CF<sub>n</sub>Cl<sub>m</sub> where
Q7: Which of the following best represents the
Q7: Instructions: Propose a structure for a molecule
Q11: Which of the following best represents the
Q13: Draw all the lone pairs (nonbonding valence
Q20: Which of the following statements is not
Q34: How many total valence electrons are represented
Q38: Instructions: Propose a structure for a molecule