Multiple Choice
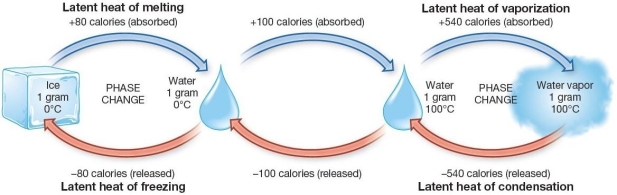 Latent Energy absorbed and released during phase changes of water
Latent Energy absorbed and released during phase changes of water
Which of the following is true regarding the evaporation of water?
A) It requires the addition of 540 cal for each gram under normal sea level pressure.
B) It requires the loss of 540 cal for each gram under normal sea level pressure.
C) It requires the addition of 100 cal for each gram under normal sea level pressure.
D) It requires the loss of 100 cal for each gram under normal sea level pressure.
E) No latent energy is released or absorbed.
Correct Answer:

Verified
Correct Answer:
Verified
Q43: Relative humidity is<br>A)the amount of water vapor
Q44: The difference between the dry adiabatic rate
Q45: Which of the following normally would be
Q46: In winter,freezing water can break pipes and
Q47: The wet adiabatic rate is_ than the
Q49: Ice pellets larger than 0.5 cm (0.20
Q50: A steel needle,though denser than water,can float
Q51: Condensation nuclei over the ocean consist primarily
Q52: High altitude wispy clouds made of ice
Q53: When temperatures are below freezing,the temperature at