Multiple Choice
A heat engine performs the reversible cycle abca with 9.0 moles of an ideal gas, as shown in the figure. Path ca is an adiabatic process. The temperatures at points a and b are 300 K and 500 K, respectively. The volume at point c is 0.20 m3. The adiabatic constant of the gas is 1.60. The thermal efficiency of this engine is closest to 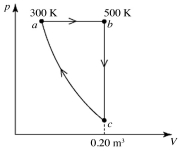
A) 0.070.
B) 0.10.
C) 0.13.
D) 0.16.
E) 0.19.
Correct Answer:

Verified
Correct Answer:
Verified
Q5: The temperature inside a Carnot refrigerator placed
Q16: A refrigerator removes heat from the freezing
Q17: According to the second law of thermodynamics,the
Q19: A Carnot cycle engine operates between a
Q20: Is it possible to transfer heat from
Q26: An automobile engine takes in 4000 J
Q29: A 2.0-kg block of aluminum at 50°C
Q34: An ice cube at 0°C is placed
Q40: A heat engine with an efficiency of
Q43: The entropy of an isolated system must