Multiple Choice
A 0.10- 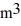 gas tank holds 5.0 moles of nitrogen gas (N2) , at a temperature of 370 K. The atomic mass of nitrogen is 14 g/mol, the molecular radius is 3.0 × 10-10 m, and the Boltzmann constant is 1.38 × 10-23 J/K. The root-mean-square speed (thermal speed) of the molecules is closest to
gas tank holds 5.0 moles of nitrogen gas (N2) , at a temperature of 370 K. The atomic mass of nitrogen is 14 g/mol, the molecular radius is 3.0 × 10-10 m, and the Boltzmann constant is 1.38 × 10-23 J/K. The root-mean-square speed (thermal speed) of the molecules is closest to
A) 570 m/s.
B) 810 m/s.
C) 410 m/s.
D) 22 m/s.
E) 99 m/s.
Correct Answer:

Verified
Correct Answer:
Verified
Q3: An ideal gas is kept in a
Q4: A cubic box with sides of 20.0
Q11: The root-mean-square speed (thermal speed)of the molecules
Q13: The root-mean-square speed (thermal speed)for a certain
Q13: Dust particles are pulverized rock, which has
Q14: Assuming the radius of diatomic molecules is
Q16: A sample of an ideal gas is
Q21: An ideal gas is kept in a
Q25: The mean free path of an oxygen
Q47: A mole of oxygen (O<sub>2</sub>)molecules and a