Multiple Choice
In a solution of acetic acid ( 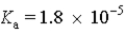 ) at physiological pH of 7.3,it is most accurate to represent this substance as:
) at physiological pH of 7.3,it is most accurate to represent this substance as:
A) CH3CO2H
B) CH3CO2-
C) CH3CO2H and CH3CO2-
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q3: Name: <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB4944/.jpg" alt="Name: " class="answers-bank-image
Q4: Name: <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB4944/.jpg" alt="Name: " class="answers-bank-image
Q5: Draw the mechanism for the preparation of
Q6: Draw: cyanoacetic acid
Q7: Which of the following is not an
Q9: Name the following substance.Atoms other than carbon
Q10: Explain the differences in acidity between p-methoxybenzoic
Q11: Draw: 2-propylpentanoic acid
Q12: Draw: 2-propenenitrile
Q13: Even though the para position is one