Multiple Choice
Exhibit 6-9
Use the reaction energy diagram below to answer the following question(s) .
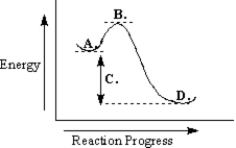
-Refer to Exhibit 6-9.The reaction depicted in this reaction energy diagram can best be described as:
A) a slow exothermic reaction
B) a fast exothermic reaction
C) a slow endothermic reaction
D) a fast endothermic reaction
Correct Answer:

Verified
Correct Answer:
Verified
Q32: Exhibit 6-7<br>Consider this reaction when answering the
Q33: Exhibit 6-10<br>Consider the reaction of 2-bromo-2-methylpropane with
Q34: Exhibit 6-10<br>Consider the reaction of 2-bromo-2-methylpropane with
Q35: What are the major differences between a
Q36: Exhibit 6-7<br>Consider this reaction when answering the
Q37: The following represents the carbocation intermediate in
Q39: Write the mechanism of the reaction of
Q40: Which of the following could act as
Q41: _ A reaction where ΔG° is negative.<br>A)transition
Q42: Exhibit 6-10<br>Consider the reaction of 2-bromo-2-methylpropane with