Multiple Choice
Consider the following energy diagram. 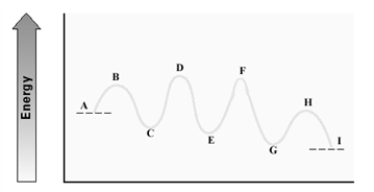 Which step has the least endergonic ΔG±?
Which step has the least endergonic ΔG±?
A) A - C
B) C - E
C) E - G
D) G - I
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q20: Exhibit 6-7<br>Consider this reaction when answering the
Q21: Consider the following grayscale electrostatic potential map.The
Q22: Consider a reaction with the following thermodynamic
Q23: Consider the following grayscale electrostatic potential map.The
Q24: _ A species that lies at an
Q26: A reaction that establishes equilibrium with almost
Q27: A reaction has ΔH° = -14.7 kJ
Q28: Exhibit 6-9<br>Use the reaction energy diagram below
Q29: Write the mechanism of the reaction between
Q30: In a polar reaction mechanism,the atom that