Multiple Choice
Consider the following energy diagram for an enzyme-catalyzed reaction. 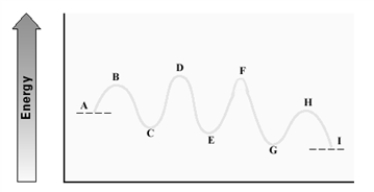 Which step is probably the slowest?
Which step is probably the slowest?
A) A - C
B) C - E
C) E - G
D) G - I
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q1: Exhibit 6-9<br>Use the reaction energy diagram below
Q3: Exhibit 6-10<br>Consider the reaction of 2-bromo-2-methylpropane with
Q4: Exhibit 6-9<br>Use the reaction energy diagram below
Q5: _ The energy needed by reactants to
Q6: Which of the following could act as
Q7: The alkane formed by hydrogenation of (S)-4-methyl-1-hexene
Q8: Write the mechanism of the reaction between
Q9: Exhibit 6-10<br>Consider the reaction of 2-bromo-2-methylpropane with
Q10: Consider the following energy diagram for an
Q11: The original question has been combined with