Multiple Choice
Consider the following energy diagram for an enzyme-catalyzed reaction. 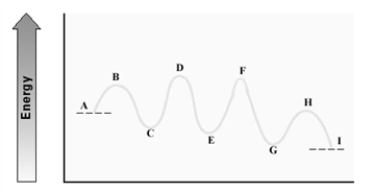 Which step has the least energetic transition state?
Which step has the least energetic transition state?
A) A - C
B) C - E
C) E - G
D) G - I
Correct Answer:

Verified
Correct Answer:
Verified
Q7: The alkane formed by hydrogenation of (S)-4-methyl-1-hexene
Q8: Write the mechanism of the reaction between
Q9: Exhibit 6-10<br>Consider the reaction of 2-bromo-2-methylpropane with
Q10: Consider the following energy diagram for an
Q11: The original question has been combined with
Q13: Consider the following process. <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB4944/.jpg" alt="Consider
Q14: The enzyme aconitase catalyzes the hydration of
Q15: Exhibit 6-10<br>Consider the reaction of 2-bromo-2-methylpropane with
Q16: Exhibit 6-10<br>Consider the reaction of 2-bromo-2-methylpropane with
Q17: Exhibit 6-9<br>Use the reaction energy diagram below