Short Answer
Exhibit 2-6
Refer to the following equation to answer the question(s) below.Place the letter corresponding to the correct answer in the blank. 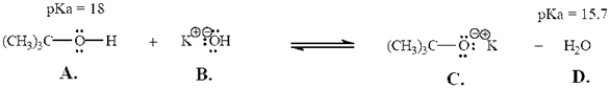
-Refer to Exhibit 2-6.The strongest Brønsted-Lowry acid in the equation is ______.
Correct Answer:

Verified
Correct Answer:
Verified
Q2: Which of the following substances has a
Q3: Exhibit 2-3<br>Phenylalanine is an amino acid that
Q4: Exhibit 2-3<br>Phenylalanine is an amino acid that
Q5: Draw the resonance forms of 3,5-heptanedione anion.<br>
Q6: Exhibit 2-9<br>Indole is pleasant smelling in highly
Q8: Exhibit 2-6<br>Refer to the following equation to
Q9: Draw two resonance structures for the species
Q10: Use the curved arrow formalism to show
Q11: Exhibit 2-10<br>Consider the acid-base reaction below to
Q12: Exhibit 2-10<br>Consider the acid-base reaction below to