Short Answer
Exhibit 2-6
Refer to the following equation to answer the question(s) below.Place the letter corresponding to the correct answer in the blank. 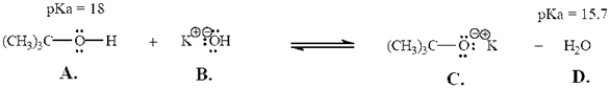
-Refer to Exhibit 2-6.The strongest Brønsted-Lowry base in the equation is ______.
Correct Answer:

Verified
Correct Answer:
Verified
Q37: An acid with a low pK<sub>a</sub>:<br>A)is a
Q38: Exhibit 2-8<br>Consider the reaction below to answer
Q39: Exhibit 2-8<br>Consider the reaction below to answer
Q40: How many resonance forms can be drawn
Q41: Exhibit 2-2<br>Calculate the formal charges on the
Q43: Exhibit 2-1<br>Give the corresponding letter of the
Q44: Exhibit 2-1<br>Give the corresponding letter of the
Q45: Exhibit 2-2<br>Calculate the formal charges on the
Q46: The structure for Vitamin K which is
Q47: Which of the following does not characterize