Multiple Choice
Uranium-238 decays into thorium-234 plus an alpha particle.How much energy is released in this process? 1 u = 931.494 MeV/c2,and the relevant mass values are 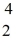 He: 4.002603 u
He: 4.002603 u 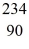 Th: 234.043583 u
Th: 234.043583 u 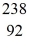 U: 238.050786 u
U: 238.050786 u
A) 4.28 MeV
B) 3.76 MeV
C) 3.18 MeV
D) 2.89 MeV
E) 5.05 MeV
Correct Answer:

Verified
Correct Answer:
Verified
Q19: A radioactive nuclide of atomic number Z
Q22: A certain nucleus containing 8 protons and
Q24: <sub>An isotope of Tc having a half-life
Q25: The following masses are known: <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB4470/.jpg"
Q26: The stability of <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB4470/.jpg" alt="The stability
Q27: A stationary plutonium-239 nucleus decays into a
Q28: The set of nuclear reactions that power
Q30: The stability of <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB4470/.jpg" alt="The stability
Q33: The carbon in your body was formed
Q72: Carbon-14 has a half-life of 5730 y.A