Multiple Choice
What is the greatest magnitude of the orbital angular momentum L for an electron in a state with principal quantum number n = 5?
A) 4.47 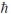
B) 4.90 
C) 5 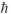
D) 5.48 
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q8: What is the minimum speed needed by
Q9: An atom has completely filled inner shells
Q15: If two electrons in an atom have
Q17: The binding energy of the hydrogen atom
Q19: What is the electron configuration for ground
Q24: An electron in a hydrogen atom has
Q26: How many electrons can be found with
Q26: How many possible sets of quantum numbers
Q44: The magnitude of the orbital angular momentum
Q46: How many electrons does it take to