Multiple Choice
A hydrogen atom is in its n = 2 excited state when its electron absorbs a photon of energy 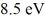 .What is the energy of the resulting free electron? The lowest level energy state of hydrogen is -13.6 eV.(h = 6.626 × 10-34 J ∙ s,1 eV = 1.60 × 10-19 J)
.What is the energy of the resulting free electron? The lowest level energy state of hydrogen is -13.6 eV.(h = 6.626 × 10-34 J ∙ s,1 eV = 1.60 × 10-19 J)
A) 5.1 eV
B) 6.6 eV
C) 6.9 eV
D) 7.7 eV
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q23: What is the orbital radius of the
Q24: Light shines through atomic hydrogen gas.It is
Q25: When a certain metal is illuminated by
Q27: In a double slit experiment,a beam of
Q29: A hydrogen atom is excited to the
Q30: A metal having a work function of
Q31: The Bohr radius of the hydrogen atom
Q32: A beam of x-rays at a certain
Q33: Light of wavelength 400 nm falls on
Q38: Suppose that in a parallel universe,the proton