Multiple Choice
A hydrogen atom makes a downward transition from the 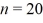 state to the n = 5 state.Find the wavelength of the emitted photon.The lowest level energy state of hydrogen is -13.6 eV. (h = 6.626 × 10-34 J ∙ s,1 eV = 1.60 × 10-19 J,c = 3.00 × 108 m/s)
state to the n = 5 state.Find the wavelength of the emitted photon.The lowest level energy state of hydrogen is -13.6 eV. (h = 6.626 × 10-34 J ∙ s,1 eV = 1.60 × 10-19 J,c = 3.00 × 108 m/s)
A) 2.43 μm
B) 1.46 μm
C) 1.94 μm
D) 2.92 μm
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q9: A beam of red light and a
Q29: A hydrogen atom is excited to the
Q30: A metal having a work function of
Q31: The Bohr radius of the hydrogen atom
Q32: A beam of x-rays at a certain
Q33: Light of wavelength 400 nm falls on
Q36: A stopping potential of 0.50 V is
Q37: How fast must a nonrelativistic electron move
Q38: Electrons emerge from an electron gun with
Q39: A hydrogen atom initially in the n