Multiple Choice
A heat engine takes 2.0 moles of an ideal gas through the reversible cycle abca,on the pV diagram shown in the figure.The path bc is an isothermal process.The temperature at c is 820 K,and the volumes at a and c are 0.010 m3 and 0.16 m3,respectively.The molar heat capacity at constant volume,of the gas,is 37 J/mol ∙ K,and the ideal gas constant is R = 8.314 J/(mol ∙ K) .The thermal efficiency of the engine is closest to 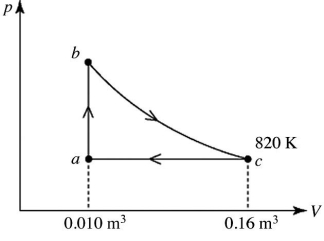
A) 0.26.
B) 0.026.
C) 0.33.
D) 0.40.
E) 0.53.
Correct Answer:

Verified
Correct Answer:
Verified
Q16: A refrigerator removes heat from the freezing
Q19: A Carnot cycle engine operates between a
Q23: A heat engine performs the reversible cycle
Q24: A Carnot engine operates between reservoirs at
Q24: An ideal Carnot engine operates between reservoirs
Q24: A certain Carnot heat pump transfers energy
Q28: The compressor in a certain Carnot refrigerator
Q29: A brass rod,75.0 cm long and having
Q31: A nuclear fission power plant has an
Q37: During each cycle of operation,a refrigerator absorbs