Multiple Choice
What is the change in entropy of 10.0 moles of ideal monatomic gas that reversibly undergoes the isothermal expansion shown in the figure? The ideal gas constant is R = 8.314 J/(mol ∙ K) . 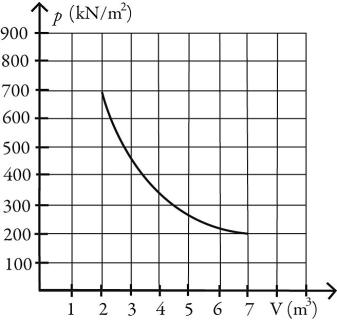
A) 221 J/K
B) 104 J/K
C) 63.1 J/K
D) 45.2 J/K
E) 90.8 J/K
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q5: The temperature inside a Carnot refrigerator placed
Q7: Is it possible to transfer heat from
Q16: A refrigerator removes heat from the freezing
Q18: A certain engine extracts 1300 J of
Q19: A Carnot cycle engine operates between a
Q22: A Carnot refrigerator takes heat from water
Q23: A heat engine performs the reversible cycle
Q24: An ideal Carnot engine operates between reservoirs
Q24: A Carnot engine operates between reservoirs at
Q45: A perfect Carnot engine operates between the