Multiple Choice
A 610-g quantity of an ideal gas undergoes a reversible isothermal compression at a temperature of 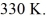 The compression reduces the volume of the gas from
The compression reduces the volume of the gas from 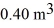 initially,to
initially,to 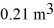 finally.The molecular mass of the gas is
finally.The molecular mass of the gas is 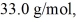 and the ideal gas constant is R = 8.314 J/(mol ∙ K) .The entropy change for the gas is closest to
and the ideal gas constant is R = 8.314 J/(mol ∙ K) .The entropy change for the gas is closest to
A) -99 J/K.
B) -81 J/K.
C) 99 J/K.
D) 81 J/K.
E) 0.00 J/K.
Correct Answer:

Verified
Correct Answer:
Verified
Q5: A real (non-Carnot)heat engine,operating between heat reservoirs
Q5: The temperature inside a Carnot refrigerator placed
Q7: The graph in the figure shows a
Q8: A 810-g quantity of ethanol,in the liquid
Q9: A Carnot engine operating between a reservoir
Q12: A 2.00-kg block of ice at 0.00°C
Q14: A Carnot engine is operated as a
Q19: What is the maximum theoretical efficiency possible
Q21: You want to design an ideal Carnot
Q23: A refrigerator has a coefficient of performance