Multiple Choice
If we double the root-mean-square speed (thermal speed) of the molecules of a gas,then
A) its temperature must increase by a factor of 4.
B) its temperature must increase by a factor of 2.
C) its temperature must increase by a factor of 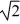 .
.
D) its pressure must increase by a factor of 2.
E) its pressure must increase by a factor of 4.
Correct Answer:

Verified
Correct Answer:
Verified
Q4: A cubic box with sides of 20.0
Q6: An oxygen molecule falls in a vacuum.From
Q8: The mean free path of an oxygen
Q11: The root-mean-square speed (thermal speed)of the molecules
Q13: A sample of an ideal gas is
Q14: What is the average kinetic energy of
Q38: What is the mean free path of
Q41: A container is filled with a mixture
Q43: The entropy of an isolated system must
Q54: Eleven molecules have speeds 16,17,18,...,26 m/s.Calculate the