Short Answer
What is the total translational kinetic energy in a test chamber filled with nitrogen (N2)at 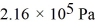 and
and 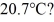 The dimensions of the chamber are
The dimensions of the chamber are 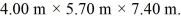 The ATOMIC weight of nitrogen is 28.0 g/mol,Avogadro's number is 6.022 × 1023 molecules/mol and the Boltzmann constant is 1.38 × 10-23 J/K.
The ATOMIC weight of nitrogen is 28.0 g/mol,Avogadro's number is 6.022 × 1023 molecules/mol and the Boltzmann constant is 1.38 × 10-23 J/K.
Correct Answer:

Verified
Correct Answer:
Verified
Q13: The second law of thermodynamics leads us
Q27: A sample of an ideal gas is
Q31: On a hot summer day,the temperature is
Q33: A 0.10- <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB4470/.jpg" alt="A 0.10-
Q34: What is the average translational kinetic energy
Q36: A hot piece of iron is thrown
Q38: At what temperature would the root-mean-square speed
Q39: A 5.0-liter gas tank holds 1.4 moles
Q48: If the temperature of a fixed amount
Q56: What is the root-mean-square value of the