Multiple Choice
The temperature of an ideal gas in a sealed 0.40 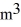 container is reduced from 400 K to
container is reduced from 400 K to 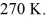 The final pressure of the gas is
The final pressure of the gas is 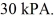 The molar heat capacity at constant volume of the gas is
The molar heat capacity at constant volume of the gas is 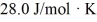 .The work done by the gas is closest to
.The work done by the gas is closest to
A) 0.00 kJ.
B) -19 kJ.
C) -25 kJ.
D) 19 kJ.
E) 25 kJ.
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q2: A cylinder contains 24.0 moles of an
Q10: The process shown in the T-V diagram
Q16: Two experimental runs are performed to determine
Q18: The filament in a light bulb has
Q19: A blacksmith is flattening a steel plate
Q20: A heat conducting rod,1.40 m long,is made
Q21: A cube at 100.0°C radiates heat at
Q22: A solid concrete wall 4.0 m by
Q28: When a gas undergoes an isothermal process,there
Q31: If 2.0 g of water at 0.00°C