Multiple Choice
Which would be true about the following reaction? M + N 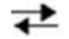 Y + Z
Y + Z
A) Adding a catalyst would alter the final concentrations of products and reactants at equilibrium.
B) Starting at chemical equilibrium, increasing the concentration of M will transiently increase the rate of formation of Y and Z.
C) Starting at chemical equilibrium, decreasing the concentration of M will increase the concentration of Y and Z.
D) Both the reaction is reversible and at chemical equilibrium and increasing the concentration of M will drive the reaction to the left are correct.
E) Because the reactants and products are different molecules, this is not a reversible reaction.
Correct Answer:

Verified
Correct Answer:
Verified
Q16: At equilibrium,in an irreversible reaction<br>A)almost all of
Q34: Glucose cannot be synthesized from fatty acids
Q38: Coenzymes are organic cofactors.
Q40: Chromosomes are composed mainly of DNA.
Q44: Living cells cannot be viewed under an
Q88: The consequences of mutations are invariably harmful.
Q103: What codon corresponds to the DNA sequence
Q104: A ribosome is composed of one molecule
Q104: During oxidative phosphorylation,hydrogen atoms are passed serially
Q127: Which describes the action of a ligand's competitive