Multiple Choice
Consider the reaction: H2CO3 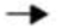 CO2 + H2O + 4 kcal/mol. Which of the following is TRUE?
CO2 + H2O + 4 kcal/mol. Which of the following is TRUE?
A) The reaction is anabolic and the energy content of the reactant is greater than that of the products.
B) The reaction is catabolic and the energy content of the reactant is greater than that of the products.
C) The reaction is anabolic and the energy content of the products is greater than that of the reactant.
D) The reaction is catabolic and the energy content of the products is greater than that of the reactant.
E) The reaction is catabolic and the energy content of the products are equal to that of the reactant.
Correct Answer:

Verified
Correct Answer:
Verified
Q2: The greater the ligand concentration needed to
Q31: Peripheral membrane proteins are involved in regulating
Q49: Free ribosomes differ from membrane-bound ribosomes in
Q53: An important function of coenzymes is to
Q57: Lysosomes are organelles specialized for breaking down
Q76: Which of the following is true concerning
Q81: One striking feature of plasma membrane structure
Q85: In skeletal muscle,when calcium binds to the
Q89: In which organelle are carbohydrate chains added
Q124: Human beings can synthesize all twenty amino