Essay
Phosphoric acid (H3PO4)has three dissociable protons,with the pKa's shown below.Which form of phosphoric acid predominates in a solution at pH 4? Explain your answer.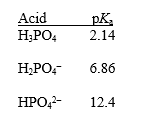
Correct Answer:

Verified
At pH 4,the first dissociable proton (pK...View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Correct Answer:
Verified
View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Q11: Explain the fact that triethylammonium chloride ((CH<sub>3</sub>CH<sub>2</sub>)<sub>3</sub>N-HCl)
Q18: A compound has a pK<sub>a</sub> of 7.4.
Q21: For each of the pairs below,circle the
Q22: Which of these statements about hydrogen
Q23: Phosphoric acid is tribasic,with pK<sub>a</sub>'s of 2.14,6.86,and
Q25: You want to maintain pH = 7.0
Q27: A true statement about hydrophobic interactions is
Q30: Three buffers are made by combining a
Q78: Define pK<sub>a</sub> for a weak acid in
Q87: The Henderson-Hasselbalch equation:<br>A) allows the graphic determination