Essay
During transfer of two electrons through the mitochondrial respiratory chain, the overall reaction is:
NADH + 1/2 O2 + H+ NAD+ + H2O
For this reaction, the difference in reduction potentials for the two half-reactions ( E'°) is +1.14 V. Show how you would calculate the standard free-energy change, G'°, for the reaction. (The Faraday constant, 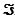 , is 96.48 kJ/V · mol.)
, is 96.48 kJ/V · mol.)
Correct Answer:

Answered by ExamLex AI
To calculate the standard free-energy ch...View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Correct Answer:
Answered by ExamLex AI
View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Q88: Which enzyme catalyzes phosphate transfer reactions between
Q89: If the <span class="ql-formula" data-value="\Delta"><span
Q90: Muscle contraction involves the conversion of:<br>A) chemical
Q91: For the following reaction, <span
Q92: Which atom is a common electrophile?<br>A) a
Q94: In the following reaction, which molecule
Q95: Which carbon atom is in the MOST
Q96: If <span class="ql-formula" data-value="\Delta"><span class="katex"><span
Q97: Which item is NOT electrophilic?<br>A) a proton<br>B)
Q98: What is the equilibrium constant for