Glycerol 3-Phosphate Dehydrogenase Catalyzes the Following Reversible Reaction:
Glycerol 3-Phosphate
Essay
Glycerol 3-phosphate dehydrogenase catalyzes the following reversible reaction:
Glycerol 3-phosphate + NAD+ NADH + H+ + dihydroxyacetone phosphate
Given the standard reduction potentials below,calculate G'° for the glycerol 3-phosphate dehydrogenase reaction,proceeding from left to right as shown.Show your work.(The Faraday constant,F ,is 96.48 kJ/V·mol. )
,is 96.48 kJ/V·mol. )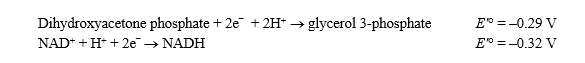
Correct Answer:

Verified
 E'° = E'° (electron acceptor)-...
E'° = E'° (electron acceptor)-...View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Correct Answer:
Verified
View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Q12: Which of the following is
Q13: E'° of the NAD<sup>+</sup>/NADH half reaction
Q13: The expression <span class="ql-formula" data-value="\Delta"><span
Q14: During glycolysis,glucose 1-phosphate is converted to
Q16: For each pair of ions or compounds
Q17: The standard reduction potentials (E'°)for the
Q18: Which of the following is true about
Q19: In glycolysis,the enzyme pyruvate kinase catalyzes
Q67: The free energy of hydrolysis of
Q96: If <span class="ql-formula" data-value="\Delta"><span class="katex"><span