Multiple Choice
An unknown compound X has the empirical formula C3H6O and a molecular ion in its mass spectrum at 116.Compound X shows no IR absorption at 3200-3600 cm-1 but shows a peak at 1700 cm-1.The 1H NMR spectral data of X is shown below.What is the structure of compound X? 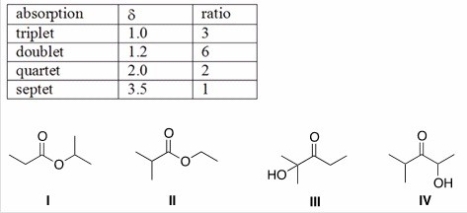
A) I
B) II
C) III
D) IV
Correct Answer:

Verified
Correct Answer:
Verified
Q35: How many different kinds of protons are
Q36: For each of the following compounds,indicate how
Q37: Which of the indicated protons absorbs further
Q38: How many different kinds of protons are
Q39: An unknown compound X has the molecular
Q41: Which of the following compounds would give
Q42: Into how many peaks will each of
Q43: Compound X has a molecular formula C<sub>8</sub>H<sub>10</sub>
Q44: How could spectroscopy be used to distinguish
Q45: How many different kinds of protons are