Multiple Choice
The base peak in a mass spectrum corresponds to the most stable fragment.Propose a structure for a compound that is consistent with the following data.
a.The molecular ion peak has m/z = 116.
b.The base peak is at m/z = 59.
c.The compound is composed of C,H and O atoms.
d.The IR spectrum shows a strong absorbance at 3257 cm-1. 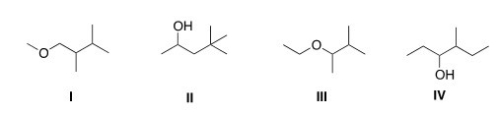
A) I
B) II
C) III
D) IV
Correct Answer:

Verified
Correct Answer:
Verified
Q6: Ignoring sp<sup>3</sup> CH stretching,estimate where the major
Q7: When the phenol shown below is treated
Q8: Examine the IR below and classify the
Q9: Which of the following statement(s)is (are)true about
Q10: Why does an alkyne carbon-carbon triple bond
Q12: Which of the following structures is consistent
Q13: Stronger bonds will be found where in
Q14: An alkyne C-H bond absorbs at higher
Q15: What type of signal(s)would you observe in
Q16: The functional group region of an infrared