Multiple Choice
Would this crossed Aldol reaction work well? Why or why not? 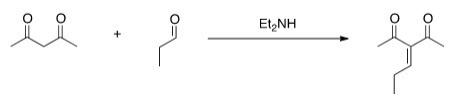
A) Yes,the diketone is significantly more acidic,so this enolate can be formed selectively.
B) Yes,the aldehyde is significantly more acidic,so this enolate can be formed selectively.
C) No,the aldehyde is significantly more acidic,so this enolate cannot be formed selectively.
D) No,the diketone is significantly more acidic,so this enolate cannot be formed selectively.
Correct Answer:

Verified
Correct Answer:
Verified
Q33: What is the general name for the
Q34: In a Michael reaction,what is the name
Q35: Of the carbonyl compounds; (1)benzaldehyde,(2)acetophenone and (3)dicyclohexyl
Q36: What is the name given to the
Q37: What is the product of the self-condensation
Q39: What reaction type is a Claisen reaction?<br>A)Electrophilic
Q40: If a Claisen condensation reaction is run
Q41: What is the major product of the
Q42: What is the general name for the
Q43: What is the general name of the